Scaling up a novel microfluidic process
We addressed the critical issues in scaling up a microfluidic process for drug production.
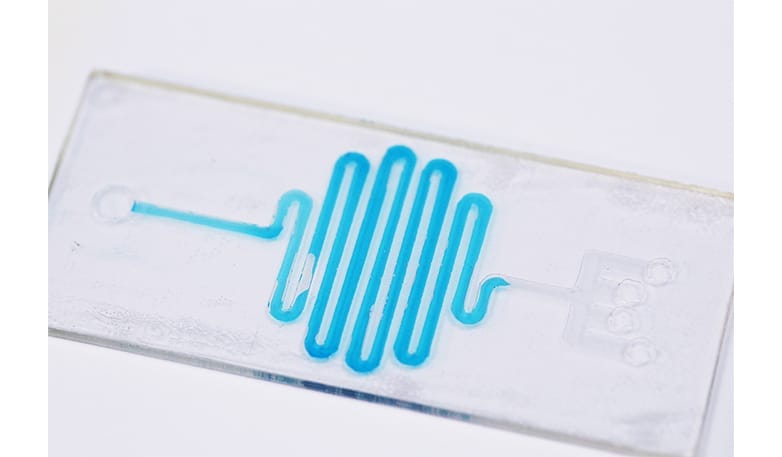
Our client is a high-tech start-up with a novel microfluidic process for encapsulating peptide and protein generics in spheres that enable controlled release of the drug. Complex issues with the physics were preventing them from scaling up small prototypes into a production-scale device
Approach
We analysed the issues from a number of angles
- We reviewed scientific literature into the microfluidic processes and identified the key parameters that lead to successful production of spheres
- We identified new device designs that eliminated geometries where fluidic problems can occur
- We developed a mathematical model to predict the sensitivity of a scaled-up, parallel process to manufacturing tolerances
This resulted in seven recommendations to guide the development process.
Benefit
- This was a short, sharp piece of work that helped our client surmount a critical obstacle
- They have now secured £2 million in funding, which will enable them to develop the scaled-up process
View the full case study here

