Array
Array
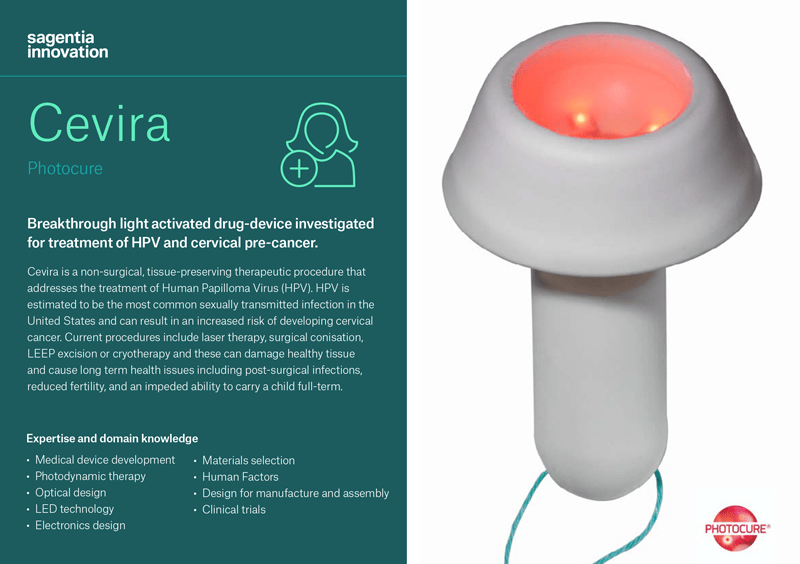
Cevira
Our client asked:
Photocure, a Norwegian specialty pharmaceutical company focused in dermatology and cancer, asked Sagentia Innovation to partner with them to develop a device that would work in combination with Photocure’s pharmaceutical as a non-surgical alternative to treat HPV and cervical pre-cancer. The goal of the project was to more effectively remove HPV infection and treat precursors of cervical cancer. Cevira is a drug-device combination procedure which delivers a targeted light-activated treatment that destroys tissue infected by HPV and treats precancerous lesions on the cervix, without damaging healthy tissue.
The project story:
Working in partnership with Photocure, we started with a technology assessment exercise to map out the most viable technology for the device and generated device concepts. A key part of the project was carrying out user research to look at form and fit – ensuring these were right early in the project meant a smoother transition to the subsequent detailed engineering phase and resulted in the best end product.
Following proof of concept, including clinical tests, and detailed design engineering, including optical design, electronics design, materials selection and design for assembly, Sagentia Innovation developed a design suitable for clinical trials.
Results: deliverables and outcomes
- Cevira was accepted for use in a Phase II clinical trial by the US FDA. The trials included 240+ patients in multiple centres across the United States and Europe.
- This is the first therapeutic treatment that uses advanced LED technology in a self-powered, disposable device which can be deployed inside a body cavity. The device contains a LED light source that in combination with a medicinal product initiates a photochemical reaction in exposed tissue.
- The fully integrated single-use device is easily administered by a trained gynaecologist or colposcopist and is then left in place on the cervix for up to 24 hours, during which time the patient is able to leave the hospital and continue with daily activities, before removing and disposing of the device themselves
- By combining recent advances in LED technology with expertise in optics, electronics and medical device development, Sagentia Innovation and Photocure have developed a viable alternative to invasive treatments that is expected to improve patient health outcomes and help reduce costs for the healthcare system
Our markets

Our consultants, scientists and engineers redefine what’s possible and help R&D groups across the medical, industrial, consumer and food and beverage sectors achieve commercial return from their opportunities.
Our projects

We have completed over 10,000 projects for start-ups and global market leaders alike, from understanding the market & technology landscape through to developing and delivering complex products.

